Cleanroom protective clothing - a barrier for bacteria?
A common method for determining the release of particles by people under working conditions is the body box test (Figure 1). Here, the wearer performs defined movements in a cabin through which clean air flows. Airborne particles that escape the filter effect pass with the air flow to a collection device, where they are quantitatively recorded. In addition to non-specific counting, the underlying standard IEST-RP-CC003.4 also provides for a differentiated determination of microbiologically relevant emissions. For this purpose, the collected particles are passed onto culture media, cultivated and counted. Both tests allow an estimation of the effectiveness of cleanroom clothing with regard to its retention capacity. However, the results are influenced by the individual characteristics of the test person as well as by particles from the protective material itself. The body box test is therefore particularly suitable for the direct comparison of different protective clothing products.
The measurement of bacterial filtration efficiency (BFE) according to DIN EN 14683 or ASTM F2101 provides more easily reproducible results on the specific retention of germs. In this test method described for face masks, a bacterial suspension is applied to the protective material as an aerosol. Test germs that penetrate the material reach culture media where they can be incubated and counted. The BFE value is the percentage of retained germs compared to test runs without protective material. Although the test promises clear key figures for quality assessment, test results for protective clothing in the pharmaceutical sector are rare. The reasons for this can be assumed to be the high equipment costs and the use of pathogenic test germs. The strict normative requirements limit the testing possibilities to a few specialised testing facilities.
In order to determine whether and how BFE tests can be used more simply, the standard test procedure was simplified in the Bern R&D Laboratory. In the process, the set-up and procedure were reduced to the essential elements without, however, fundamentally changing the test principle. Modifications concerned the technical equipment for nebulising and collecting the bacteria (Figure 2), the type of germs used (Bacillus subtilis instead of Staphylococcus aureus) and the level of bacterial load. The latter was increased from the standard value (2.2 x 103 CFU per test) to up to 1.7 x 105 CFU per test, which allowed better differentiation of the test results.
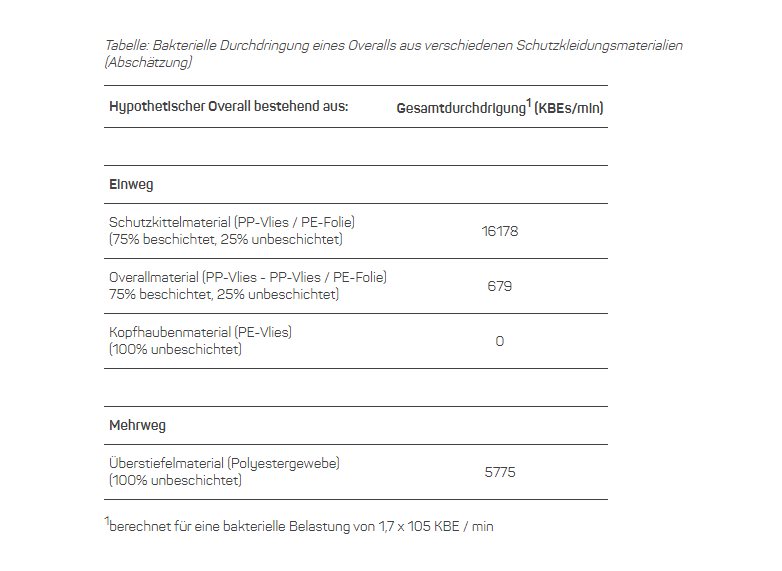
The modified test method was used to examine typical cleanroom protective materials as used in the aseptic preparation of medicinal products. The selection consisted of a reusable (overboots) and various disposable materials (coveralls, gowns, arm sleeves, head bonnet). If the latter had both breathable and liquid-tight (plastic-coated) zones, both areas were sampled and tested. The measured values allowed a direct comparison of the bacterial filtration performance, but were also used to estimate the total penetration of a hypothetical overall made of the respective materials or material mixtures. This made it possible to compare the total filter performance for the most important piece of protective clothing in this context under identical conditions of use.
It came as little surprise that all plastic-coated materials effectively prevented the penetration of liquids. The filter performance of uncoated protective materials proved to be more variable and thus more relevant for the final result. For example, the breathable gown material had a surprisingly low penetration resistance, while the other uncoated disposable materials offered high filter performance. The retention capacity of the reusable fabric was significantly higher than that of the breathable gowning material, but could not match the effectiveness of most disposable materials. Transferring the individual measurement results to the hypothetical overall underlines the differences in performance and allows direct conclusions to be drawn about the hygienic effectiveness of a whole garment (table).
Consequences
The demanding purity requirements of the ApBetrO for the aseptic preparation of medicinal products require not only a high technical effort, but also the use of protective clothing suitable for cleanrooms. The suitability of the products offered in a variety of models and materials is usually difficult to assess because meaningful test results are lacking or not provided. The user is therefore dependent on the (advertising) statements of the manufacturers when choosing the right protective clothing. In addition to tests in the body box, testing the bacterial filter performance can provide reliable results for quality assessment. The test results presented here show that there are significant differences in the important aspect of germ retention. Even though the tests have so far only been carried out on an exemplary basis, it can already be seen that there is no such thing as the right material (disposable or reusable, (partially) coated or uncoated). Manufacturers are therefore required to provide clear evidence of performance based on individual test results.
A comprehensive report on the tests carried out and the results was published in issue 4/2016 of the journal "Onkologische Pharmazie" of the German Society for Oncological Pharmacy (DGOP).

 Deutsch
Deutsch
 English
English
.jpg)