Expertise GMP Annex 1 in pharmacies
Quo vadis, safety cabinet?
With the revised GMP Annex 1, the question of the correct containment technology for the manufacture of patient-specific parenterals is moving more into focus.
While RABS and isolators are largely regarded as having no alternative in industrial pharmaceutical production, safety cabinets in accordance with DIN 12980 represent a practicable and safe alternative in the area of patient-specific drug preparation. Safety cabinets in accordance with DIN 12980 not only offer an excellent level of product protection, but also highly effective occupational safety when handling hazardous substances.
New challenges for pharmacies and manufacturing companies
The new GMP Annex 1 requires that all critical manufacturing steps take place under cleanroom class A conditions if final sterilisation in the final container is not possible - for example when preparing medicinal products with CMR properties. The selection of the right containment system is therefore a decisive factor for product safety, regulatory compliance and, last but not least, occupational health and safety.
RABS and isolators - the only way?
The new GMP Annex 1 makes recommendations: Where possible, systems such as RABS or isolators should be used. These technologies physically separate the aseptic work area from the environment and minimise the risk of manual intervention. Accordingly, many consider them to be the only permissible solution - especially in the pharmaceutical industry. However, this view falls short, especially in the context of patient-specific preparation in pharmacies. Here, flexibility, practicality and personal safety are just as important as product safety.
Safety cabinets according to DIN 12980: An underestimated standard
Modern ‘safety cabinets for cytostatics’ (SfZ), certified in accordance with DIN 12980, not only fulfil the requirements for product protection in accordance with GMP purity class A. They also offer tested and documented personal protection when handling CMR drugs. They also offer tested and documented personal protection when handling CMR drugs.
Safety cabinets impress with
- Balanced air flow with robust air curtain,
- highly efficient particle filtration of the supply and exhaust air (HEPA filter),
- documented testing of personal protection in accordance with official standards,
- ergonomic, flexible working even with discontinuous production.
This makes them a practical, safe and economically attractive solution - especially when ready-to-use customised preparations have to be produced regularly.

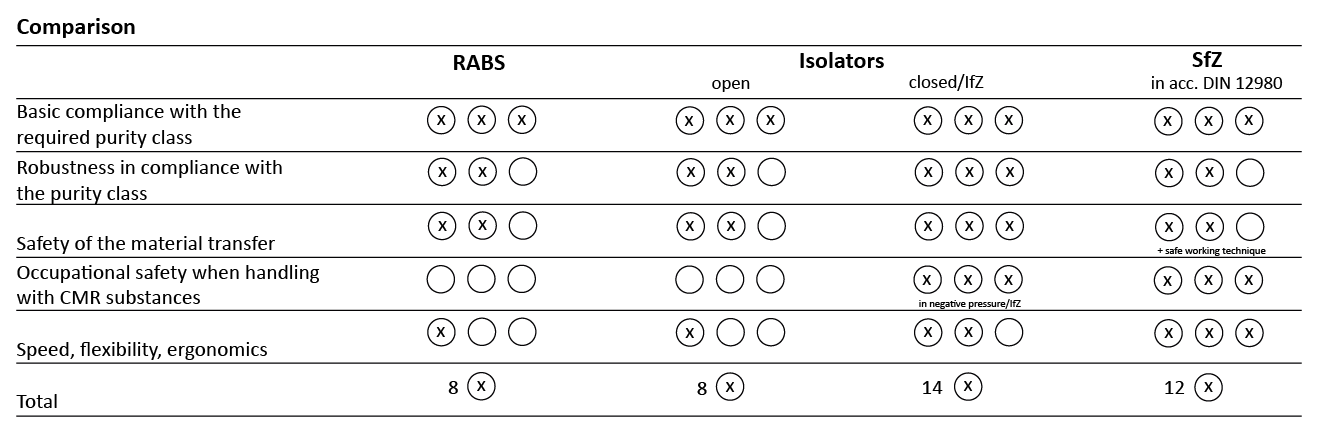
System comparison at a glance A direct comparison of the containment systems shows:
- RABS and open isolators offer a high level of product protection, but no tested occupational safety and only poor ergonomics.
- Closed isolators / isolators for cytostatics (IfZ) offer the highest level of product and personal protection, but require a great deal of effort to use in practice and are therefore often impractical for customised patient preparation.
- Safety cabinets (SfZ) perform particularly well in terms of ergonomics, flexibility and practicality - while offering a high level of protection.
Our conclusion: safety cabinets as a viable solution - also in the GMP environment
In normal pharmacy operations, safety cabinets are still the gold standard - and quite rightly so: they combine legal occupational safety with aseptic manufacturing quality and are established, proven and recognised.
Safety cabinets in accordance with DIN 12980 are also a permissible and sensible solution in GMP-regulated manufacturing plants - provided that their use is justified and embedded in a suitable contamination control strategy. The responsible supervisory authority should be involved in the decision-making process at an early stage.
Especially where flexibility, personal protection and economical operation are required, safety cabinets offer the prerequisite for a realistic, practice-orientated implementation of the new GMP requirements.

 Deutsch
Deutsch
 English
English

